Research - (2023) Volume 13, Issue 2
Crustacean (Malacostraca) plankton assemblages in the northern Bay of Bengal: A comparison of seasonal and geographical patterns
J. Mawa, S. Rana, N. Sultana and Sk.A. Al-Nahid*Abstract
The production of wild crustacean larvae is crucial to Bangladesh's emerging shellfish industry. In the coastal zone of the northern Bay of Bengal, Bangladesh, environmental parameters and geographical and seasonal distribution patterns of the Malacostracan plankton stage (Subphylum: Crustacea) were studied. Plankton samples were collected from the surface using a bongo net. During the sampling period, four groups were identified: shrimp larvae, krill, crab zoea, and mantis shrimp larvae. Among them, almost 63% of krill were recorded, followed by shrimp larvae (21.1%), crab zoea (12.4%), and mantis shrimp larvae (3.4%). In addition, krill was the most abundant group at each station and season. One-way ANOVA was conducted to identify significant variation (P<0.05) in the mean larval abundance of crab zoea, shrimp larvae, krill, and mantis shrimp larvae based on the season of each group. A significant difference was observed among shrimp larvae, crab zoea, and mantis shrimp larvae during the season. However, krill abundance was not significantly different between seasons. Univariate analysis of correlations between stations and the abundance of crustacean groups was performed. Pairwise comparisons of mean anbundance showed significant variations between the identified four groups and three stations (Naf, Bakkhali and Rezu Khal estuaries). However, only the mean abundance of krill varied significantly with all the four stations (P<0.05). These results suggest that the abundance of Malacostracan crustaceans did not depend on stations; rather, their abundance varied significantly within seasons in the northern Bay of Bengal.
Keywords
Malacostraca, Plankton, Distribution pattern, Univariate analysis, Estuaries.
Introduction
Aquatic crustaceans have a two-stage life cycle: a benthic juvenile-adult stage and a pelagic larval stage. The pelagic larval phase in the life cycle of benthic crustaceans is critical for settling or dispersing in the appropriate location (Anger, 2006). There are over 42,000 known species of Crustacea, making them one of the largest extant groups and greatly contributing to the diversity of life on Earth (Bowman and Abele, 1982). Approximately 90% of the Decapoda of crustaceans reside in or near aquatic habitats such as sea or brackish waters (Kaestner, 1980; Anger, 2001). The largest Crustacea group, Malacostraca (superior crustaceans), comprises more than 40,000 species, with approximately 15,000 documented decapods (De Grave, et al., 2009) and 25,000 peracids (Ahyong, et al., 2011). It covers well-known creatures, such as woodlice, amphipods, mysids (Peracarida), shrimps, lobsters, crayfish, crabs (Decapoda), krill (Euphausiacea), and several smaller and lesser-known species (Štrus, 2019).
The vast majority of marine and terrestrial decapod species have a complex life history: instead of growing from the egg to a benthic juvenile, they create pelagic larvae that may be radically different in shape and behavior from the juvenile and adult members of the same species. Biphasic life cycles are evolutionarily ancient features of many metazoans, including Crustacea (Rieger, 1994). Crustacean larvae feed on coexisting plankton creatures in the water column, unlike adults, which float in estuaries, coastal areas, or ocean currents (Anger, 2001). Larval assemblages are abundant in coastal areas exclusively in low saline environments to avoid difficulties with osmoregulation, as larvae are more susceptible to high saline environments (Koettker and Freire, 2006). Marine reserves can be created to support ongoing efforts to preserve biological diversity while enhancing fishing yields and protecting the particularly vulnerable life stages of marine organisms (Palumbi, 2003). Data related to larval distribution, dispersal, and abundance are critical tools for sustainable management of a focused organism (Sale and Kritzer, 2003).
Bangladesh is part of the Indo-Malayan ecozone and borders the Bay of Bengal for approximately 710 km (Das and van Dijk, 2013). Bangladesh contains an extremely diverse range of fish and shrimp species. Shamsuddoha and Islam (2017) discovered 442 marine finfish and 36 shellfish in Bangladesh's marine and estuarine waterways. More than 50 species of commercially significant crustaceans and 301 mollusks have been identified in the Bay of Bengal and coastal areas so far (Akter, et al., 2017). In addition, 31 different species of turtles, 15 different types of crabs, three lobsters, and 25 tortoises can be found in Bangladesh's coastal waters (IUCN, 2000; Quader, 2010). A recent report from Bangladesh's inland and coastal waters identified 63 shrimp and prawn species. Although Penaeus monodon Fabricius, 1798 is the target species because of its export value, the brown shrimp, Metapenaeus monoceros Fabricius, 1798, accounts for approximately 56% of the overall shrimp capture (Fatema, et al., 2022). Peneaus monodon, P. semisulcatus var. exsulcatus Hilgendorf, 1879, P. indicus H. Milne Edwards, 1837, Metapeneaus monoceros, M. brevocornis H. Milne Edwards, 1837 are important penaeids. According to economists, Bangladesh's shrimp and prawn industry has grown in importance, contributing considerably to the country's foreign exchange revenues and employment generation in rural regions. This sector employs approximately 600,000 people along the coast (Quader, 2010; Rashid, 2019).
In the current study, a benchmark survey spanning a year was carried out in Bangladesh's northern Bay of Bengal, although the detection level of crustaceans is high and focuses mostly on the major decapod groups rather than the species level. Various studies have been conducted on fish and shrimp species (Rashed-Un-Nabi, et al., 2011; Parvez, et al., 2018) and zooplankton communities (Iqbal, et al., 2014; Abdullah Al, et al., 2020) in Cox's Bazar region, Bangladesh. However, there is limited evidence regarding larvae and macrozooplankton in the crustacean group. Therefore, establishing a comprehensive understanding of their distribution and abundance in coastal regions and how they react to changing ecological and abiotic factors can help ensure the sustainable management of their fisheries. This study explored the seasonal distribution of crustacean larvae and their use as a nursing ground.
Materials and Methods
Selection of the sampling station
The research was conducted for 12 months at four (4) chosen research stations in the Cox's Bazar-Teknaf coasts in the northern Bay of Bengal, including the Bakkhali Estuary (N 21.471501, E 91.950445), Rezu Khal estuary (N 21.2952777, E 92.035000), Moheshkhali para (N 20.8636944, E 92.250555), and Naf estuary (N 20.730300, E 92.3421011) (Fig. 1).
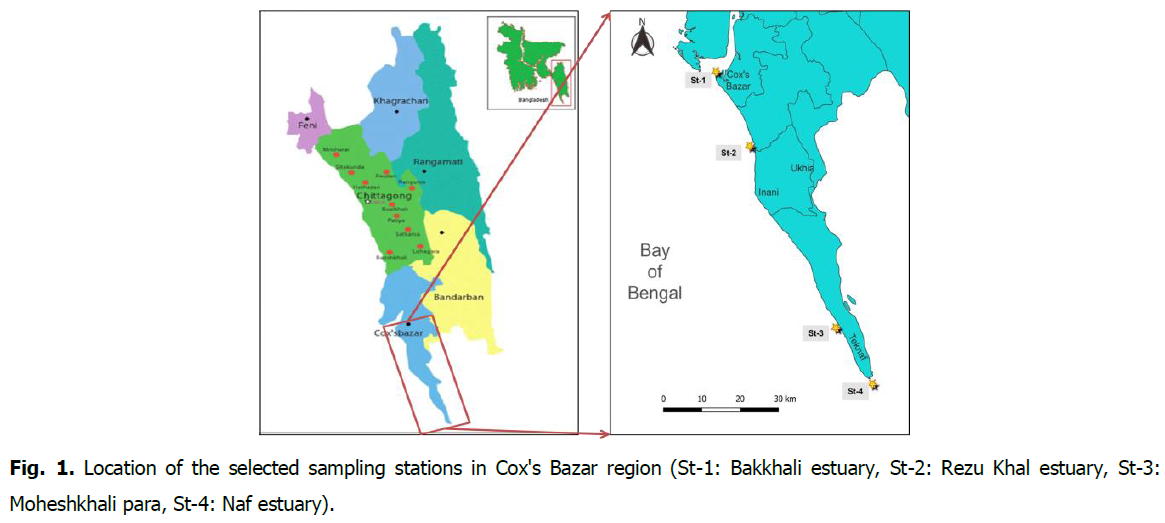
Fig 1. Location of the selected sampling stations in Cox’s Bazar region (St-1: Bakkhali estuary, St-2: Rezu Khal estuary, St-3: Moheshkhali para, St-4: Naf estuary).
The Bakkhali River and Rezu Khal originate in India, flow through Bandarban and Cox's Bazar district, and fall into the Bay of Bengal. The Naf River and Moheshkhali para are situated in Teknaf Upazila, in the southeasterly region of Bangladesh. The research station map was constructed using ArcGIS (version 10.8.1) based on GPS coordinates (version 5.07).
Crustacean and water sample collection
Sampling was conducted monthly from January 2021 to December 2021 at all four stations. A bongo net with a mouth diameter of 0.50 m, a length of 1.3 m, and a mesh size of 500 m was applied. Each tow covered approximately 2 km of the surface area and lasted 10 min during daylight. To measure the amount of seawater filtered during each haul, a digital flow meter (KC Denmark A/S 23.090-23.091) was connected to the mouth of the net. Following every tow, the samples were immediately fixed in 90% ethanol and transported to the laboratory. Data from water parameters and crustacean groups were divided into three seasons to summarize temporal variation: winter (November to February), pre-monsoon (March to May), and monsoon (June to October) (Parvez, et al., 2018). A long, weighted tube was used to collect water samples to obtain integrated samples.
Crustacean sample enumeration and identification
The amount of water (m3) filtered through the net was used to calculate the seasonal change in water discharge, and the following equation was used to determine the water flow rate (Abdullah Al, et al., 2020):
Indicated number of revolutions (FR-IR) × impeller pitch × net opening area (m2) × 1000
Where, FR=Final reading, IR=Initial reading, Pitch of the impeller=0.3, Net opening area,
2πr2=2 × 3.1416 × 0.252
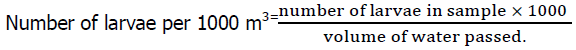
Crustacean larvae were sorted from the entire sample for taxonomic identification. They were identified under a stereo microscope (OPTIKA Microscope Italy C-B3) at low magnification (10 x) using the descriptions of related taxa provided by Sterrer (1986), Lynne (2004), and Sanet, et al., (2006). Individuals were counted over the entire sample to complete enumeration. Abundance was expressed as the number of individuals per 1000 cubic meters (individual.1000m-3).
Environmental parameters analysis
During the sampling process, in-situ hydrological parameters were assessed at each sampling site. Salinity, pH, temperature, Dissolved Oxygen (DO), Total Dissolved Solids (TDS), and transparency were determined using a refractometer (HANNA HI-96822), pH meter (HANNA H198107), centigrade thermometer, DO meter (HANNA DO200A), TDS meter (HACH 720072), and Secchi disc, respectively. The total alkalinity of the water samples was measured in the laboratory using the gram titration method, where Phenolphthalein and Methyl Orange were used as indicators.
Statistical analysis
Univariate Pearson’s correlation analysis was performed using SPSS (version 25) to identify any variance in individual abundance between seasons and stations. Two-way ANOVA was conducted to determine the relationships among sampling stations, larval groups, and abundance. A residual analysis was performed to confirm the presumptions of two-way ANOVA. Outliers were determined by inspection of a boxplot, normality was determined for each design cell using the Shapiro-Wilk normality test, and homogeneity of variances was determined using Levene's test. There were no outliers, the residuals were normally distributed (P>0.05), and the variances were homogeneous. All pairwise comparisons were run for each variables with reported 95% confidence intervals and Bonferroni-adjusted P-values within each. One-way ANOVA was performed to determine the mean and standard deviation of the collected water parameters.
Results
Spatial and seasonal patterns of environmental parameters
The spatial variations in the environmental parameters of the northern Bay of Bengal, Bangladesh, from January to December (2021) are presented in Table 1. The highest mean salinity (ppt) was observed in Moheshkhali para (26.83 ± 5.93). The salinity ranged between 8 and 34 ppt in the Naf estuary, 9-34 ppt in the Bakkhali estuary, and 13-34 ppt in the Rezu Khal estuary. The value of pH was mostly similar in all four stations, with a mean of 8.22 ± 0.26 (Bakkhali estuary), 8.26 ± 0.44 (Naf estuary), 8.27 ± 0.21 (Moheshkhali para) and 8.28 ± 0.59 (Rezu Khal estuary). Dissolved oxygen (DO) values were the highest in Moheshkhali para (7.20 ± 0.82 ppm) and lowest in Naf estuary (6.71 ± 1.16 ppm). The maximum temperature was recorded at 33°C in Moheshkhali para, and the minimum was 18.80°C in the Naf estuary. The highest mean TDS value was found in the Naf estuary (484.17 ± 86.52 ppm), and the lowest was found in the Bakkhali estuary (450.83 ± 52.46 ppm). The transparency value ranged from 48 to 106 cm in Moheshkhali para, 43-93 cm in the Naf estuary, 27-95 cm in the Bakkhali estuary, and 38-126 cm in the Rezu Khal estuary. The mean alkalinity (ppm) was recorded 113.67 ± 41.03, 130.08 ± 58.49, 125.42 ± 46.46 and 126.17 ± 54.34 in Moheshkhali para, Naf estuary, Bakkhali estuary, and Rezu Khal estuary, respectively.
| Station | Salinity (ppt) | pH | DO (ppm) | Temp. (°C) | TDS (ppm) | Trasparency (cm) | Alkalinity (ppm) | |
|---|---|---|---|---|---|---|---|---|
| Moheshkhali para | Mean ± SD | 26.83 ± 5.93 | 8.27 ± 0.21 | 7.20 ± 0.82 | 28.25 ± 3.21 | 465.08 ± 118.78 | 72.42 ± 20.56 | 113.67 ± 41.03 |
| Max | 36 | 8.50 | 8.32 | 33 | 655 | 106 | 229 | |
| Min | 18 | 7.80 | 5.7 | 23.9 | 320 | 48 | 74 | |
| Naf estuary | Mean ± SD | 23.81 ± 8.55 | 8.26 ± 0.44 | 6.71 ±1.16 | 27.35 ± 3.88 | 484.17 ± 86.52 | 63.33 ± 19.63 | 130.08 ± 58.49 |
| Max | 34 | 9.1 | 8.16 | 32 | 622 | 93 | 256 | |
| Min | 8 | 7.3 | 3.78 | 18.80 | 355 | 43 | 52 | |
| Bakkhali estuary | Mean ± SD | 24.81 ± 7.03 | 8.22 ± 0.26 | 7.04 ± 0.88 | 28.35 ± 2.96 | 450.83 ± 52.46 | 60.08 ±22.44 | 125.42 ± 46.46 |
| Max | 34 | 8.8 | 9.08 | 31.5 | 545 | 95 | 204 | |
| Min | 9 | 7.9 | 5.6 | 23 | 395 | 27 | 59 | |
| Rezu khal estuary | Mean ± SD | 24.97 ±6.76 | 8.28 ±0.59 | 6.96 ±0.74 | 27.81 ± 2.62 | 475.25 ± 74.34 | 62.58 ± 25.82 | 126.17 ± 54.34 |
| Max | 34 | 8.80 | 7.9 | 30.7 | 622 | 126 | 258 | |
| Min | 13 | 6.50 | 5.30 | 22.7 | 375 | 38 | 65 |
Table 1. Spatial variations in mean physico-chemical parameters such as salinity, pH, DO, temperature, TDS, transparency and Alkalinity of northern Bay of Bengal, Bangladesh.
Seasonal variations in the hydrological parameters during the study period are shown in Table 2. Mean salinity (ppt) was recorded at 19.04 ± 5.73, 27.39 ± 2.98 and 32.17 ± 3.27 in rainy, winter and pre-monsoon, respectively. Winter had the highest mean pH value of 8.29 ± 0.20 and in post monsoon the range was comperatively largest. Dissolved oxygen (DO) ranged from 3.78 to 8.1 ppm in winter, from 5.28 to 7.56 ppm in pre-monsoon and from 5.3 to 9.08 ppm in rainy monsoon. The highest mean temperature (°C) was recorded in the pre-monsoon (30.68 ± 1.39) and the lowest was recorded in winter (26.06 ± 2.87). The rate of TDS was between 390 and 655 ppm in winter, 320 and 488 ppm in pre-monsoon, and 376 and 618 ppm in the rainy monsoon. The highest Secchi disc depth was recorded at 126 cm in the pre-monsoon season and the lowest was 27 cm in the rainy monsoon season. The mean alkalinity (ppm) was determined 158.69 ± 54.85, 121.17 ± 22.80 and 97.55 ± 39.73 in winter, pre-monsoon, and rainy monsoon, respectively. One-way ANOVA showed significant seasonal variations in salinity, temperature, TDS, transparency, and alkalinity during the sampling year.
| Season | Salinity (ppt) | pH | DO (ppm) | Temp. (°C) | TDS (ppm) | Transparency (cm) | Alkalinity (ppm) |
|
|---|---|---|---|---|---|---|---|---|
| Winter | Mean ± SD | 27.39 ± 2.98 | 8.29 ± 0.2 | 7.11 ± 0.97 | 26.06 ± 2.87 | 513.38 ± 87.72 | 54.31 ± 8.32 | 158.69 ± 54.85 |
| Min | 21 | 7.9 | 3.78 | 22.7 | 390 | 39 | 100 | |
| Max | 32.5 | 8.5 | 8.1 | 30.6 | 655 | 71 | 258 | |
| Pre monsoon | Mean ± SD | 32.17 ± 3.27 | 8.22 ± 0.16 | 6.60 ± 0.75 | 30.68 ± 1.39 | 394.58 ± 47.54 | 91.75 ± 16.39 | 121.17 ± 22.8 |
| Min | 24 | 8 | 5.28 | 28.65 | 320 | 65 | 100 | |
| Max | 36 | 8.6 | 7.56 | 33 | 488 | 126 | 170 | |
| Rainy monsoon | Mean ± SD | 19.04 ± 5.73 | 8.25 ± 0.57 | 7.10 ± 0.91 | 27.80 ± 2.97 | 477.75 ± 71.16 | 56.55 ± 19.16 | 97.55 ± 39.73 |
| Min | 8 | 6.5 | 5.3 | 18.8 | 376 | 27 | 52 | |
| Max | 26 | 9.1 | 9.08 | 31.1 | 618 | 93 | 201 |
Table 2. Seasonal variations in mean physico-chemical parameters such as salinity, pH, DO, temperature, TDS, transparency and alkalinity of northern Bay of Bengal, Bangladesh.
Composition and abundance of the identified crustacean groups by station
The collected specimens were sorted, counted, and identified, and then seasonal and station-wise data analyses were performed. The mean abundance of the groups was 67.8184 per 1000 m3, with the highest percentage of crustaceans being krill (63.1%), followed by shrimp larvae (21.1%), crab zoea (12.4%), and mantis shrimp larvae (3.4%). Fig. 2 shows that the mean abundance of krill was high at each station. However, in the Rezu Khal estuary, krill abundance was highest at 281 individuals.1000 m-3. The mean numbers of shrimp larvae, crab zoea, and mantis shrimp larvae at this station were 57, 30, and 11 per 1000 m3. The highest number of shrimp larvae and crab zoea were obtained, with 64 and 41 individuals per 1000 m3 from the Naf and Bakkhali estuaries, respectively. Mantis shrimp larvae were mostly found in the Naf and Rezu Khal estuaries (11 individuals.1000 m-3).
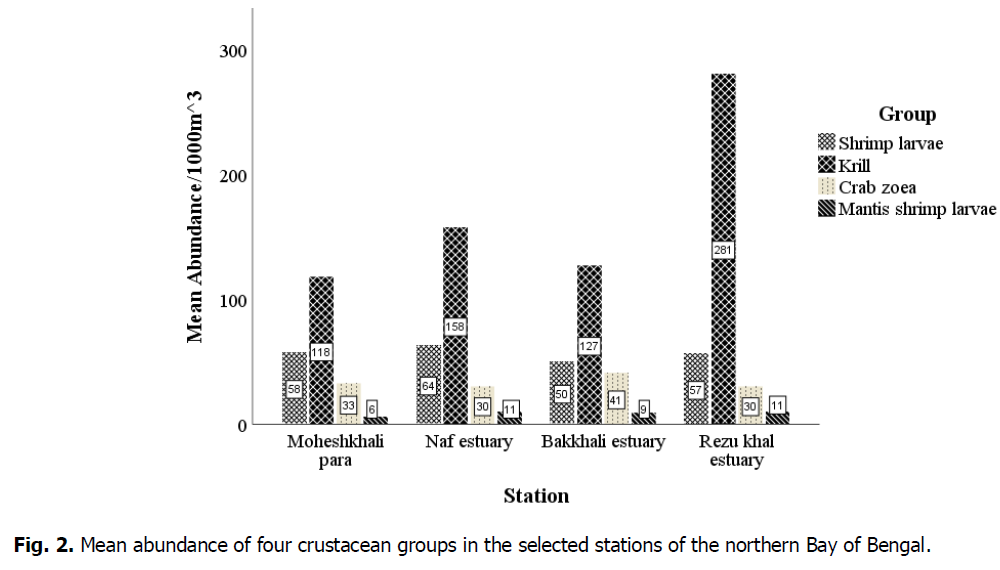
Fig 2. Mean abundance of four crustacean groups in the selected stations of the northern Bay of Bengal.
Seasonal variation of the abundance of the crustacean groups
Krill was highest in pre-monsoon (179 individuals.1000m-3) and lowest in the rainy monsoon season (162 individuals.1000m-3). Shrimp larvae were abundant in the rainy monsoon (90) and gradually decreased during the pre-monsoon (43) and winter (28). Crab zoea and mantis shrimp larvae followed the same trend (Fig. 3).
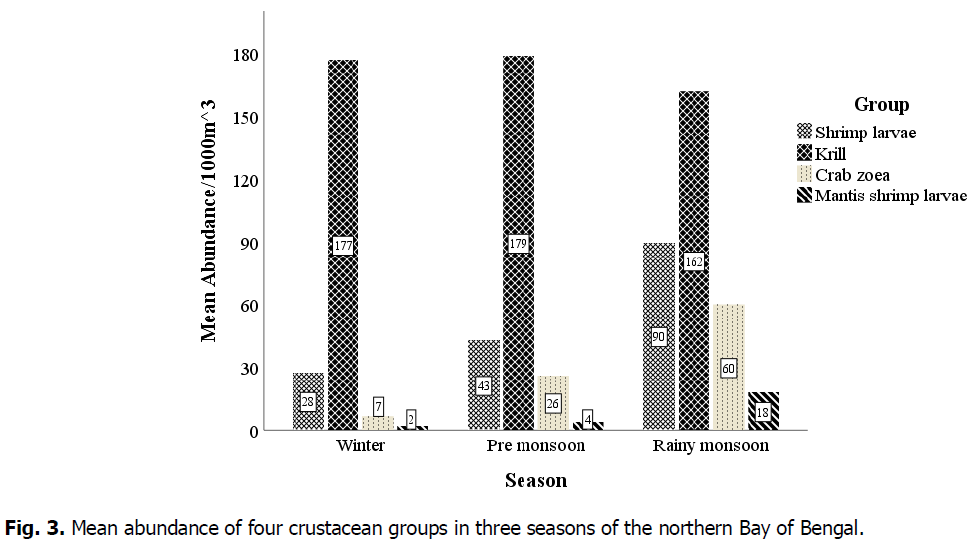
Fig 3. Mean abundance of four crustacean groups in three seasons of the northern Bay of Bengal.
One-way analysis of variance (ANOVA) was conducted to identify significant variation (P<0.05) in the mean larval abundance of crab, shrimp, krill, and mantis shrimp based on the season of each group. Data normality was analyzed using the Shapiro-Wilk test (P>0.05) for each group. The seasons were classified into three categories: winter (n=16), pre monsoon (n=12), and rainy monsoon (n=20). The larval abundance of shrimp was significantly different among seasons (F=5.323, P=0.008). Abundance (individual.1000 m-3) increased from winter (27.5 ± 25.01) to pre monsoon (42.89 ± 34.81) to rainy monsoon (89.53 ± 84.73). The abundance of crab zoea was significantly different between the seasons (F=0.012, P<0.05). Significant differences were observed in the mantis shrimp larvae during the season (F=43.924, P<0.05). However, no significant difference was observed between the krill and sampling seasons (P=0.988) (Table 3).
| Group | Season | Abundance (individual.1000m-3) |
|---|---|---|
| Shrimp larvae | Winter | 27.5 ± 25.01a |
| Pre monsoon | 42.89 ± 34.81ab | |
| Rainy monsoon | 89.53 ± 84.73b | |
| Krill | Winter | 169.94 ± 205.58a |
| Pre monsoon | 179 ± 224.317a | |
| Rainy monsoon | 167.42 ± 186.86a | |
| Crab zoea | Winter | 6.56 ± 5.087a |
| Pre monsoon | 26.05 ± 9.409b | |
| Rainy monsoon | 60.05 ± 26.28c | |
| Mantis shrimp larvae | Winter | 2.02 ± 3.3a |
| Pre monsoon | 3.97 ± 3.047a | |
| Rainy monsoon | 17.9495 ± 7.65b |
Table 3. Abundance of four crustacean groups showing significant differences among the seasons.
Station and season wise variations among the groups
Significant interactions between larval groups and their abundances were observed during the sampling period, F (3,48)=22.912, P<0.05, partial η2=0.281. However, there were no combined effects of the stations and groups on mean larval abundance. The mean abundance of crustaceans in the Moheshkhali para group was not significantly different from each other. However, krill-crab zoea-mantis shrimp larvae in the Naf estuary were significantly different (P<0.05). In addition, the mean abundance of krill-mantis shrimp larvae varied significantly within the Bakkhali estuary. In the Rezu Khal estuary, the mean abundances of shrimp larvae, krill, crab zoea, and mantis shrimp larvae differed significantly (P<0.05). A significant difference was observed in the mean abundance of krill at all the selected stations throughout the sampling period (P<0.05) (Table 4).
| Station | Group | Mean ± SD | P value |
|---|---|---|---|
| Naf estuary | Krill | 158.17 ± 122.05 | 0.018 |
| Crab zoea | 30.36 ± 23.26 | 0.018 | |
| Manta shrimp larvae | 10.55 ± 12.59 | 0.004 | |
| Bakkhali estuary | Krill | 127.22 ± 202.91 | 0.036 |
| Manta shrimp larvae | 9.33 ± 10.04 | 0.036 | |
| Rezu Khal estuary | Shrimp larvae | 56.58 ± 73.33 | <0.001 |
| Krill | 281.25 ± 266.61 | <0.001 | |
| Crab zoea | 30.39 ± 24.41 | <0.001 | |
| Manta shrimp larvae | 10.53 ± 7.30 | <0.001 |
Table 4. Pairwise comparison of mean abundance between stations and groups by Bonferroni adjustment.
Discussion
In this study, variations in environmental parameters, such as salinity, pH, DO, temperature, TDS, transparency, and alkalinity, were observed at four stations in the northern Bay of Bengal in three seasons covering a year (2021). There were significant seasonal variations in the salinity, temperature, TDS, transparency, and alkalinity during the sampling year. As three of the stations (Bakkhali, Rezu Khal, and Naf) were in the estuary and Moheshkhali para was in a coastal environment, salinity and transparency varied due to rainfall and river discharge. A study conducted by Abdullah Al, et al. (2020) in the northern Bay of Bengal, Bangladesh, confirmed that the TDS value was highest in winter due to mining and discharge from fish and shrimp hatcheries and saltpans, which is consistent with the present findings.
Although environmental parameters and zooplankton distribution have previously been used to evaluate the water quality and ecological stability of ecosystems worldwide (Ferdous and Muktadir, 2009; Liu, et al., 2013; Srichandan, et al., 2013, 2015), they have not yet been well documented in the coastal waters of Bangladesh, the northern Bay of Bengal. In this study, four crustacean larval groups- shrimp larvae, crab zoea, krill, and mantis shrimp larvae-were prioritized and identified up to the family level. Almost all research studies conducted in this region covered ichthyoplankton and zooplankton communities. As these identified crustacean groups are considered zooplankton, the discussion section focuses on them.
Iqbal, et al. (2014) identified 12 groups of zooplankton in the Bakkhali Estuary, including shrimp larvae and crab zoea. Das, et al. (1982) identified 21 zooplankton communities on the continental shelf of the Bay of Bengal. Sharif (2002) identified 23 major zooplankton groups from the Meghna River estuary, while Goswami (1985) identified 24 zooplankton taxa, namely Copepoda, Cladocera, Crab larvae, Acetes H. Milne Edwards, 1837 (shrimp), Isopoda, Mysids, Fish larvae, Amphipoda, Fish eggs, Shrimp larvae, and some other zooplankton from the coastal waters of Goa.
Krill was the highest crustacean group obtained in this study, which was observed mostly in pre monsoon season, when salinity, TDS, and transparency peaked. A study on krill in the Indian Ocean reported that species richness was low in the Arabian Sea and Bay of Bengal, but average taxonomic distinction (AvTD) was highest in the Bay of Bengal (Sutton and Beckely, 2015). The Bay receives a significant freshwater inflow from rivers draining the subcontinent (Subramanian 1993), which lowers the salinity compared to other parts of the Indian Ocean and increases nutrient uptake, creating a suitable environment for krill.
The highest percentage of crustaceans in this study was observed in the rainy monsoon, which is similar to Sharif, et al. (2017), who reported 37 major zooplankton groups with maximum occurrences in the monsoon from the Meghna River estuary, Bangladesh. In the northern Bay of Bengal, Abdullah Al, et al. (2020) also determined the highest diversity in the monsoon season, approximately 38 holo and meroplankton species. Iqbal, et al. (2014) detected the highest abundance in winter in the Bakkhali estuary. However, the lowest percentage was observed in both studies during pre monsoon. Fazeli, et al. (2013) reported 15 groups of zooplankton in the Oman Sea with peak abundance observed in the northeast monsoon period, which outlined how changes in species distribution and composition could be caused by changes in water hydrographic conditions.
Shrimp larvae were highest during the rainy monsoon season (June to October) during the study period. Generally, P. monodon PL is abundant from October to February and is associated with moderate salinity (Hoq, et al., 2001). However, Abdullah Al, et al. (2020) reported that the seasonal monsoon is highly diversified when Acetes, shrimps, mysids, crab zoea, and fish larvae are more abundant than in other groups. Primavera (1998) and Basu, et al., (1998) observed two peaks for penaeid recruitment and settlement in winter and pre monsoon at average salinity and high temperature.
Univariate statistical analysis of the correlations between stations and abundance of crustacean groups was performed. Among the four stations, three (Naf, Bakkhali, and Rezu Khal estuary) were significantly correlated with the abundance of shrimp larvae, krill, crab zoea, and mantis shrimp larvae (P<0.05). Furthermore, univariate statistical analysis of the correlations between crustacean groups and abundance was performed. Among the four groups, only one (krill) was significantly correlated with all four stations (P<0.05).
Conclusion
In conclusion, this study targeted the identification of Malacostracan (Subphylum: Crustacea) plankton in the northern Bay of Bengal, Bangladesh, as well as their spatial and seasonal distribution along the coastline. Environmental parameters were also analyzed, and significant seasonal variations were observed in salinity, temperature, TDS, transparency, and alkalinity. Krill abundance was highest in the premonsoon period, while shrimp larvae, crab zoea, and mantis shrimp larvae were mostly detected in the rainy monsoon season. In addition, the Rezu Khal estuary had the highest abundance, indicating that it was the most suitable nursery ground on this coast. As the shellfish industries of Bangladesh are mostly dependent on the wild catch of Malacostracan larvae, this investigation will give a clear concept of the present scenario of this specific taxon.
Acknowledgement
The authors are greatful to the Ecological Laboratory of Chattogram Veterinary and Animal Sciences University for laboratory support.
References
Al, M.A., Akhtar, A., Rahman, M.F., AftabUddin, S., Modeo, L. (2020). Temporal distribution of zooplankton communities in coastal waters of the northern Bay of Bengal, Bangladesh. Regional Studies in Marine Science, 34:100993.
Ahyong, S.T., Lowry, J.K., Alonso, M., Bamber, R.N., Boxshall, G.A., Castro, P., Svavarsson, J. (2011). Subphylum Crustacea Brünnich, 1772. In: Zhang, Z.-Q.(Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148:165-191.
Akter, T., Hossain, M.M., Begum, R., Barman, P.P., Debnath, P.K. (2017). Diversity of fish species in south-eastern coast of Bangladesh. Journal of the Sylhet Agricultural University, 4:267-279.
Anger, K. (2001). The biology of decapod crustacean larvae. Lisse: AA Balkema Publishers, 14:1-420.
Anger, K. (2006). Contributions of larval biology to crustacean research: a review. Invertebrate Reproduction and Development, 49:175-205.
Basu, S., Saha, S.B., Niyogi, S., Mitra, A., Choudhury, A. (1998). Population dynamics of crustacean juveniles in the Hooghly estuary near Sagar Island, West Bengal. Environment and Ecology, 16:616-622.
Bowman, T.E., Abele, L.G. (1982). Classification of the recent Crustacea. The Biology of Crustacea, 1:1-27.
Das, N.G., Bhuiyan, A.L., Miah, W. (1982). Taxonomy of a calanoid copepode Candacia catula (Giesbrecht) from north-eastern region of the Bay of Bengal. Mahasagar.
Das, I., van Dijk, P.P. (2013). Species richness and endemicity of the herpetofauna of South and Southeast Asia. Raffles Bulletin of Zoology.
De Grave, S., Pentcheff, N.D., Ahyong, S.T., Chan, T.Y., Crandall, K.A., Dworschak, P.C., Wetzer, R. (2009). A classification of living and fossil genera of decapod crustaceans. Raffles Bulletin of Zoology.
Fatema, U.K., Faruque, H., Salam, M.A., Matsuda, H. (2022). Vulnerability Assessment of Target Shrimps and Bycatch Species from Industrial Shrimp Trawl Fishery in the Bay of Bengal, Bangladesh. Sustainability, 14:1691.
Google Scholar, Crossref, Indexed at
Fazeli, N., Savari, A., Nabavi, S.M.B., Zare, R. (2013). Seasonal variation of zooplankton abundance, composition and biomass in the Chabahar Bay, Oman Sea. International Journal of Aquatic Biology, 1:294-305.
Ferdous, Z., Muktadir, A.K.M. (2009). A review: potentiality of zooplankton as bioindicator.
Goswami, S.C. (1985). Zooplankton standing stock and composition in coastal waters of Goa, west coast of India.
Hoq, M.E., Islam, M.N., Kamal, M., Wahab, M.A. (2001). Abundance and seasonal distribution of Penaeus monodon postlarvae in the Sundarbans mangrove, Bangladesh. Hydrobiologia, 457:97-104.
Iqbal, M.M., Islam, M.S., Haider, M.N. (2014). Heterogeneity of zooplankton of the Rezukhal Estuary, Cox’s Bazar, Bangladesh with seasonal environmental effects. International Journal of Fisheries and Aquatic Studies, 2:275-282.
IUCN. (2000). International Union of Conservation of Nature Bangladesh, Red Data Book List of Bangladesh, pp:24.
Kaestner, A. (1980). Invertebrate Zoology. Crustacea Krieger. Huntington, New York, p:35.
Koettker, A.G., Freire, A.S. (2006). Spatial and temporal distribution of decapod larvae in the subtropical waters of the Arvoredo archipelago, SC, Brazil. Iheringia. Série Zoologia, 96:31-40.
Liu, D., Keesing, J.K., He, P., Wang, Z., Shi, Y., Wang, Y. (2013). The world's largest macroalgal bloom in the Yellow Sea, China: formation and implications. Estuarine, Coastal and Shelf Science, 129:2-10.
Lynne, M.W. (2004). Aquatic Invertebrate Taxonomist, Cooperative Freshwater Ecology Unit.
Palumbi, S.R. (2003). Population genetics, demographic connectivity, and the design of marine reserves. Ecological Applications, 13:146-158.
Parvez, M.M., Billah, M.M., Iqbal, M.M., Rahman, M.M., Bhuiyan, M.K.A., Romkey, S.S., Islam, M.S. (2018). Fish diversity and water characteristics in the Reju khal river estuary, Bangladesh. Water Conservation and Management (WCM), 2:11-19.
Primavera, J.H. (1998). Mangroves as nurseries: shrimp populations in mangrove and non-mangrove habitats. Estuarine, Coastal and Shelf Science, 46:457-464.
Quader, O. (2010). Coastal and marine biodiversity of Bangladesh (Bay of Bengal). In Proceeding of International Conference on Environmental Aspects of Bangladesh (ICEAB10), Japan.
Rashed-Un-Nabi, M., Al-Mamun, M.A., Ullah, M.H., Mustafa, M.G. (2011). Temporal and spatial distribution of fish and shrimp assemblage in the Bakkhali river estuary of Bangladesh in relation to some water quality parameters. Marine Biology Research, 7:436-452.
Rashid, S.M.A. (2019). Coastal biodiversity-a review. Report prepared for long term monitoring research and analysis of Bangladesh coastal zone.
Rieger, R.M. (1994). The biphasic life cycle-a central theme of metazoan evolution. American Zoologist, 34:484-491.
Sale, P.F., Kritzer, J.P. (2003). Determining the extent and spatial scale of population connectivity: decapods and coral reef fishes compared. Fisheries Research, 65:153-172.
Sanet, J.V., Jonathon, T., Carin, V.G., Annelise, G. (2006). Easy identification of the common fresh algae: A guide for the identification of microscopic algae in Southern fresh waters, North West University, Potchefsroom, Pretoria.
Shamsuddoha M., Islam, M.M. (2017). Bangladesh national conservation strategy: Coastal and marine resources. Department Forest and International Union for Conservation of Nature, p:50.
Sharif, A.S.M. (2002). A comparative study on plankton and benthos of the Meghna river-estuary during monsoon and post monsoon.
Sharif, A.S.M., Bakar, M.A., Bhuyan, M.S. (2017). Assessment of water quality of the lower Meghna river estuary using multivariate analyses and RPI. International Journal of Chemical and Pharmaceutical Technology, 2:57-73.
Srichandan, S., Panda, C.R., Rout, N.C. (2013). Seasonal distribution of zooplankton in Mahanadi estuary (Odisha), east coast of India: a taxonomical approach. International Journal of Zoological Research, 9:17.
Srichandan, S., Kim, J.Y., Bhadury, P., Barik, S.K., Muduli, P.R., Samal, R.N., Rastogi, G. (2015). Spatiotemporal distribution and composition of phytoplankton assemblages in a coastal tropical lagoon: Chilika, India. Environmental Monitoring and Assessment, 187:1-17.
Štrus, J., Žnidaršič, N., Mrak, P., Bogataj, U., Vogt, G. (2019). Structure, function and development of the digestive system in malacostracan crustaceans and adaptation to different lifestyles. Cell and Tissue Research, 377:415-443.
Subramanian, V. (1993). Sediment load of Indian rivers. Current Science, pp:928-930.
Sutton, A.L., Beckley, L.E. (2022). Krill along the 110° E meridian: Oceanographic influences on assemblages in the eastern Indian Ocean. Deep Sea Research Part II: Topical Studies in Oceanography, 202:105133.
Author Info
J. Mawa, S. Rana, N. Sultana and Sk.A. Al-Nahid*Citation: Mawa, J., Rana, S., Sultana, N., Al-Nahid, Sk.A. (2023). Crustacean (Malacostraca) plankton assemblages in the northern Bay of Bengal: A comparison of seasonal and geographical patterns. Ukrainian Journal of Ecology. 13:1-10.
Received: 12-Jan-2023, Manuscript No. UJE-23-86924; , Pre QC No. P-86924; Editor assigned: 14-Jan-2023, Pre QC No. P-86924; Reviewed: 26-Jan-2023, QC No. Q-86924; Revised: 01-Feb-2023, Manuscript No. R-86924; Published: 08-Feb-2023, DOI: 10.15421/2023_423
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
